Japan has a fully integrated plan to advance and accelerate regenerative medicine products with safety and efficacy. If successful, this will be a boon for humankind. At the same time it represents a challenge to the international community, and especially the United States, to keep up with Japan’s forward progress.
Japan’s plan is robust and transparent (you need only look) and includes the development of the International Strategic Zone of Kanagawa Prefecture at Tonomachi, Kawasaki City as a key component. The biomedical cluster in Kanagawa includes a huge and growing complex of enormous buildings, housing both laboratories and offices. The area is directly across the river from Tokyo International Airport (Haneda.) A planned bridge spanning the two will soon reduce the drive time to 5 minutes.
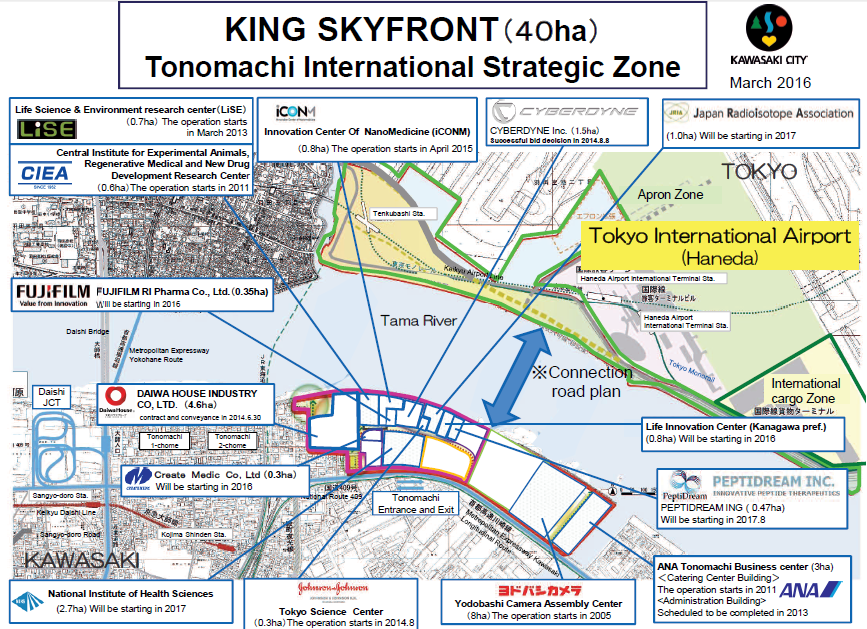
Already located in the Strategic Zone are the Japan National Institute of Health Science (NIHS), the Central Institute for Experimental Animals (CIEA), Johnson & Johnson Tokyo Science Center, Cyberdyne, FujiFilm and Innovation Center of Nano Medicine, among others.
On August 2016 I was fortunate to have a briefing and tour of the Strategic Zone, including the brand new Life Innovation Center, specifically dedicated to the rapid industrialization of regenerative medicine. Among its first
tenants are Cellular Dynamics, Scottish Development International, Ricoh, Takara Bio, and Wako Pure Chemical Industries.
The Life Innovation Center describes itself in as a “one-stop service framework, where activities to realize practical application and industrialization of regenerative medicine and cell therapy are seamlessly conducted, including R&D, clinical research, quality control and delivery of products. Expected tenants would be industries and their supporting businesses which aim at marketing their products by producing cells on contract with domestic and overseas customers, or by utilizing the early pharmaceutical approval system of regenerative medicine products.”
We at Regenerative Medicine Foundation are following closely these exciting and meaningful developments. In fact at our main annual event, the upcoming World Stem Cell Summit, we will have a strong Japan focus and interest –including a 5th annual Japanese Symposia, and abundant presence of industry, regulatory and academic leaders throughout the programming. This is a testament to our belief that understanding Japan’s commitment to rapid commercialization of stem cell therapies may be a source of key insights for the whole regenerative medicine field.

