Vancouver, Canada, August 7, 2019 – ISCT, the International Society for Cell and Gene Therapy, the global professional society of clinicians, researchers, regulatory specialists, technologists and industry partners in the cell and gene therapy sector, today announces its objection to linking the benefits of cellular immunotherapy, in particular Chimeric Antigen Receptor (CAR) T-cell therapy, with recent market offerings for third party cryopreserving and banking of T-cells for future therapeutic use.
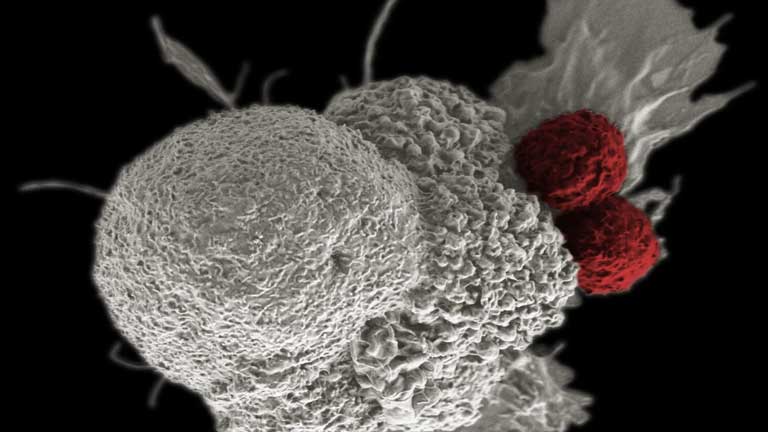
Companies around the world have launched, or may consider launching, services offering to freeze donor’s T-cells for future therapeutic use. This service is marketed to members of the public that might consider themselves potential future patients requiring chimeric antigen receptor T-cell (CAR-T) therapies. CAR-T therapies are derived from the patient’s own T-cells, the body’s immunity cells, and genetically modified to attack and destroy cancer cells whilst leaving normal cells untouched when re-administered to the patient.
ISCT, as a leading global organization representing all stakeholders across the cell and gene therapy field, has led and coordinated direct action to combat and raise awareness about unethical and unproven cell and gene therapies. This includes collaborating with more than 30 scientific societies and institutions globally. It first discussed unproven cell and gene therapies with the FDA in 2012. Direct action has ranged from establishing a multilateral task force, implementing a long term program of regulatory harmonization, to publishing a reference guide for the patient community, translated in 11 languages and authored by 23 leading figures in the field.
“Currently, companies are promising an undeliverable service. The commercial use of CAR-T cells employs the collection procedure of apheresis to remove billions of white blood cells for further processing. If and when a commercial product is developed in the future that could utilize as few cells as could be obtained from a small blood draw, there would be extensive quality and regulatory requirements for CAR-T therapy providers to use pre-stored T-cells from a third party banking service. Marketing this service today as a prelude to a potential therapy in the future to cancer patients, and especially to healthy people, is misleading,” said Bruce Levine, PhD, President-Elect, ISCT. “As one of the inventors of CAR-T therapies, after working on Kymriah, the first CAR-T product from Novartis, I find it highly unlikely that current commercial manufacturers would assent to using a third party bank of blood drawn cells in the near future.”
ISCT expresses concern about companies offering to freeze cells for future CAR-T use for several of the following reasons:
Despite the recent headlines of breakthrough CAR-T therapy in specific cancer treatments, these therapies are only used after other conventional cancer treatments, such as chemotherapy, are exhausted. Today, CAR-T therapy has only been approved for a distinct subset of blood cancers. There is therefore a low probability of patients that acquire cancer actually being eligible for CAR-T therapy. There is an even lower probability that healthy people, even those with a relative with cancer, would one day be eligible for CAR-T therapy.
Marketing claims for cell banking (and other similar services) must be balanced and objective. In the view of ISCT, the marketing and promotional materials by companies offering T-cell preservation services are currently misrepresentative of the practical use of the banked cells.
Blood and cell banks need to be qualified and audited through the proper regulatory frameworks. Current methods of commercial manufacture of CAR-T cell therapies require collection of billions of cells through a procedure of apheresis. In the judgement of ISCT there is currently no likelihood that a small blood draw of cells collected by third party banking services may be suitable for use by commercial manufacturers of CAR-T cell therapies.
“Patients and members of the public need to have the right information available to make informed decisions on using services to preserve healthy T-cells,” said Massimo Dominici, MD, Chair of the ISCT Presidential Task Force on the Use of Unproven and/or Unethical Cell & Gene Therapy, ISCT. “Without scientific validation, these companies are creating the ground for “frozen” unproven cell and gene therapies, potentially generating disappointing and even harmful results for the field.”
About ISCT
Established in 1992, ISCT, the International Society for Cell and Gene Therapy is a global society of clinicians, regulators, researchers, technologists and industry partners with a shared vision to translate cell and gene therapy into safe and effective therapies to improve patients’ lives worldwide.
ISCT is the global leader focused on pre-clinical and translational aspects of developing cell and gene-based therapeutics, thereby advancing scientific research into innovative treatments for patients. ISCT offers a unique collaborative environment that addresses three key areas of translation: Academia, Regulatory and Commercialization. Through strong relationships with global regulatory agencies, academic institutions and industry partners, ISCT drives the advancement of research into standard of care.
Comprised of over 1,500 cell and gene therapy experts across five geographic regions and representation from over 50 countries, ISCT members are part of a global community of thought leaders and organizations invested in the translation of cell and gene therapy research. For more information about the society, key initiatives and upcoming meetings, please visit: