Durham, NC – Results of a clinical trial released today in STEM CELLS Translational Medicine indicates that breast augmentation in patients treated with fat grafts enriched with autologous adipose-derived stem cells (ASCs) had significantly superior results compared to those treated with non-enriched grafts.
The clinical trial, conducted by a multi-institutional group of researchers led by Stig-Frederik T. Kølle, M.D., Ph.D., chief surgeon in the Department of Plastic Surgery at Aleris Hamlet Hospitals in collaboration with the stem cell research and production facility Stemform (Copenhagen), was designed to examine whether ex vivo-expanded ASC-enriched fat grafts for breast augmentation could improve the results of conventional lipofilling while offering a safe procedure. It is the first study to investigate the effect of expanded ASCs on large-volume fat grafting in a clinically relevant setting.

Stig-Frederik T. Kølle, M.D., Ph.D
“The repair of disfiguring volume defects of the female breast following cancer resection and congenital anomalies are secondary to the aging process and breast feeding represents a large and growing patient population with an increasing demand for natural solutions to address their surgical needs,” Dr. Kølle said. “Silicone implants are the gold standard for correcting breasts aesthetically, but they can have significant complications, and some have been associated with lymphoma. With the goal of an aesthetic and natural appearance, patient safety and satisfaction, an alternative strategy for breast augmentation and correction is autologous fat grafting.”
Autologous fat is gaining acceptance as an ideal soft tissue filler because it is biocompatible, versatile, non-immunogenic, and readily available. Also, harvesting fat through liposuction involves minimal trauma. However, clinical outcomes have varied extensively, and reported retention rates for transferred fat range from 25 to 80 percent, regularly necessitating repeated procedures.
Methods to increase graft retention are needed to make the procedure a reliable and attractive alternative to implants, especially in slim patients with limited fat resources. In this respect, cell enrichment has been shown to have promise. Animal and human studies have demonstrated that enrichment with ex vivo-expanded ASCs markedly improved the residual volume and histological appearance of fat grafts.
In the study reported here, 12 healthy women with small breasts who had a desire for augmentation were divided into two groups. Six received ASC-enriched fat grafts, while the control group (also six patients) received conventional, non-enriched fat grafts. The patients were followed for a minimum of 18 months and will also be given a five-year follow-up safety evaluation.
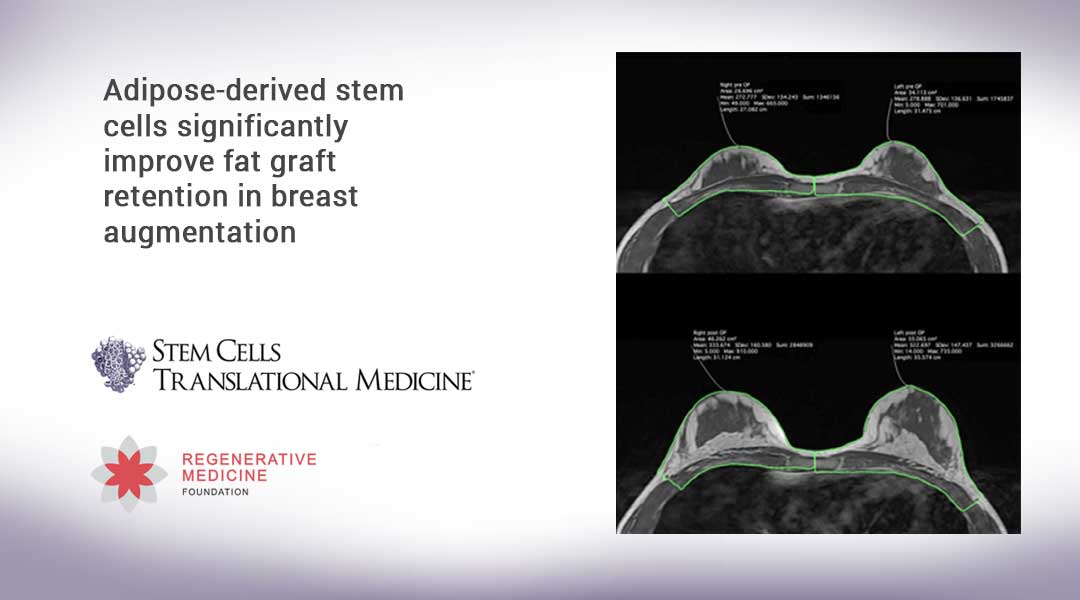
Total breast volume was determined by MRI both preoperatively and again after four months. Clinical results were also evaluated by 10 independent board-certified plastic surgeons. Photos of the patients before the operation, at four months and 18 months after the operation, were presented blinded and at an equal profile to each surgeon and assessed by the question: “If you performed one fat transplantation for breast augmentation, how satisfied would you be with the retention/survival and the cosmetic result?”
“Both the MRI and the plastic surgeons’ assessments showed significantly better clinical results in the ASC-treated group than in the controls receiving non-enriched fat grafts,” Dr. Kølle reported. “With a median survival of 80.2 percent of the total injected volume in the ASC group (compared to 45.1 percent in the control group), and a median enlargement of 2.6 times the initial breast volume, no second augmentation procedure was needed.
“This study demonstrates a safe profile for the usage of adipose-derived stromal cells,” he added. “The results are likely transferable to most soft tissue augmentations and may, therefore, be beneficial to a broad spectrum of patients.”
“The outcomes of this first clinical trial comparing breast augmentation in patients treated with fat grafts enriched with adipose-derived stromal cells to those treated with fat grafts alone are encouraging and indicate this procedure is safe and effective,” said Anthony Atala, M.D., Editor-in-Chief of STEM CELLS Translational Medicine and director of the Wake Forest Institute for Regenerative Medicine. “Such procedures could someday offer a valid alternative for patients who currently are treated with silicone breast implants.”
###
The full article, “Ex vivo-expanded autologous adipose tissue-derived stromal cells ensure enhanced fat graft retention in breast augmentation: a randomized controlled clinical trial,” can be accessed at https://stemcellsjournals.onlinelibrary.wiley.com/doi/abs/10.1002/sctm.20-0081.
About STEM CELLS Translational Medicine: STEM CELLS Translational Medicine (SCTM), co-published by AlphaMed Press and Wiley, is a monthly peer-reviewed publication dedicated to significantly advancing the clinical utilization of stem cell molecular and cellular biology. By bridging stem cell research and clinical trials, SCTM will help move applications of these critical investigations closer to accepted best practices. SCTM is the official journal partner of Regenerative Medicine Foundation.
About AlphaMed Press: Established in 1983, AlphaMed Press with offices in Durham, NC, San Francisco, CA, and Belfast, Northern Ireland, publishes two other internationally renowned peer-reviewed journals: STEM CELLS® (http://www.StemCells.com), celebrating its 38th year, is the world’s first journal devoted to this fast paced field of research. The Oncologist® (http://www.TheOncologist.com), also a monthly peer-reviewed publication, entering its 25th year, is devoted to community and hospital-based oncologists and physicians entrusted with cancer patient care. All three journals are premier periodicals with globally recognized editorial boards dedicated to advancing knowledge and education in their focused disciplines.
About Wiley: Wiley, a global company, helps people and organizations develop the skills and knowledge they need to succeed. Our online scientific, technical, medical and scholarly journals, combined with our digital learning, assessment and certification solutions, help universities, learned societies, businesses, governments and individuals increase the academic and professional impact of their work. For more than 200 years, we have delivered consistent performance to our stakeholders. The company’s website can be accessed at http://www.wiley.com.
About Regenerative Medicine Foundation (RMF): The non-profit Regenerative Medicine Foundation fosters strategic collaborations to accelerate the development of regenerative medicine to improve health and deliver cures. RMF pursues its mission by producing its flagship World Stem Cell Summit, honouring leaders through the Stem Cell and Regenerative Medicine Action Awards, and promoting educational initiatives.