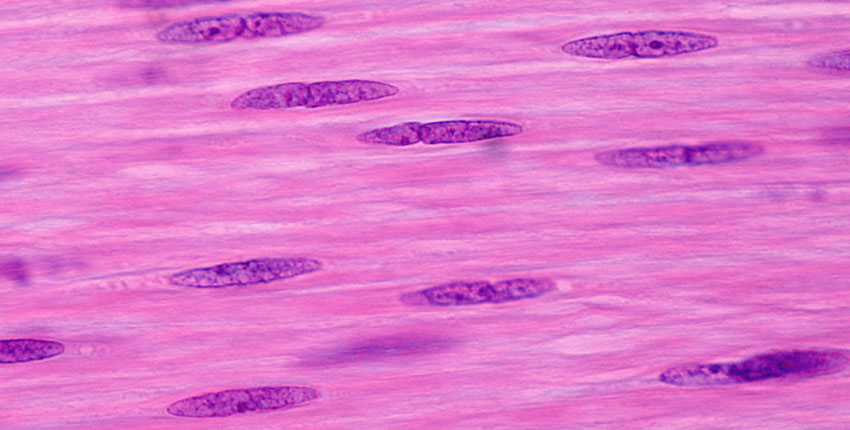
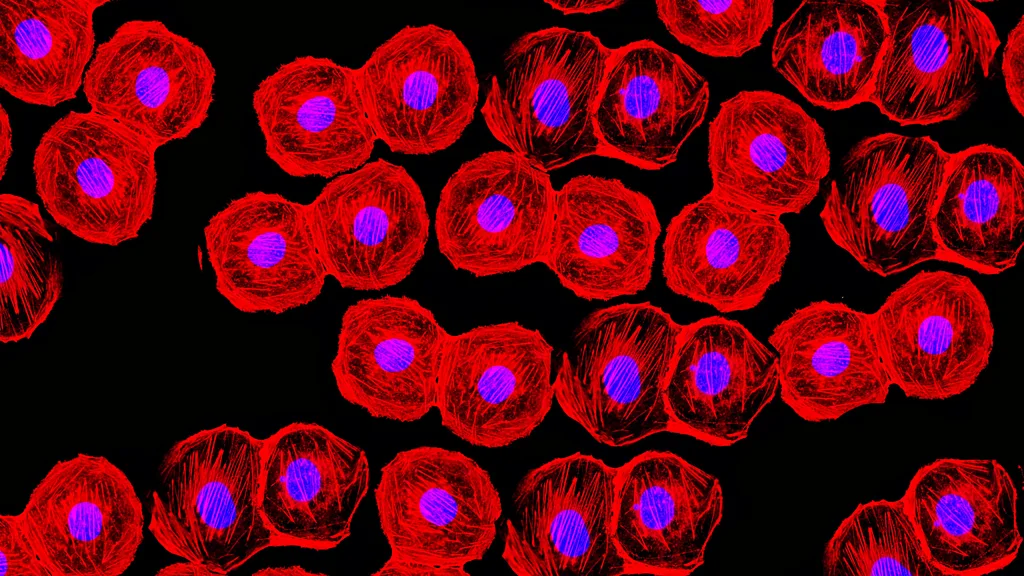
– Investigators who previously developed a recipe for turning skin cells into primitive, musclelike cells that can be maintained indefinitely in the lab without losing the potential to become mature muscle have now uncovered how this recipe works and what molecular changes it triggers within cells.
The research, led by Harvard Medical School scientists at Massachusetts General Hospital and published in Genes & Development, could allow clinicians to generate patient-matched muscle cells to help treat muscle injuries, aging-related muscle degeneration, or conditions such as muscular dystrophy.
It’s known that expression of a muscle regulatory gene called MyoD is sufficient to directly convert skin cells into mature muscle cells; however, mature muscle cells do not divide and self-renew, and therefore they cannot be propagated for clinical purposes.
“To address this shortcoming, we developed a system several years ago to convert skin cells into self-renewing muscle stemlike cells we coined induced myogenic progenitor cells, or iMPCs. Our system uses MyoD in combination with three chemicals we previously identified as facilitators of cell plasticity in other contexts,” explained senior author Konrad Hochedlinger, a professor of genetics at HMS and a principal investigator at the Center for Regenerative Medicine at MGH.
In this latest study, Hochedlinger and his colleagues uncovered the details behind how this combination converts skin cells into iMPCs.
They found that while MyoD expression alone causes skin cells to take on the identity of mature muscle cells, adding the three chemicals causes the skin cells to instead acquire a more primitive stem cell-like state.
Importantly, iMPCs are molecularly highly similar to muscle tissue stem cells, and muscle cells derived from iMPCs are more stable and mature than muscle cells produced with MyoD expression alone.
“Mechanistically, we showed that MyoD and the chemicals aid in the removal of certain marks on DNA called DNA methylation,” said lead author Masaki Yagi, a research fellow at Mass General.
“DNA methylation typically maintains the identity of specialized cells, and we showed that its removal is key for acquiring a muscle stem cell identity,” Yagi said.
Hochedlinger noted that the findings may be applicable to other tissue types besides muscle that involve different regulatory genes.
Combining the expression of these genes with the three chemicals used in this study could help researchers generate different stem cell types that closely resemble a variety of tissues in the body.
The study was funded by the National Institutes of Health, Massachusetts General Hospital, and the Gerald R. and Darlene Jordan Chair in Regenerative Medicine.