– Alofisel (darvadstrocel) is the first expanded human allogeneic adipose-derived mesenchymal stem cell therapy to be approved in Japan
– Alofisel provides a potential cell-mediated closure option for patients with complex perianal fistulas associated with Crohn’s disease who have shown an inadequate response to at least one existing medicinal treatment1,2,3
Osaka, Japan, September 27 – Takeda Pharmaceutical Company Limited (TSE:4502/NYSE:TAK) (“Takeda”) today announced that it has received approval from the Japan Ministry of Health, Labour and Welfare to manufacture and market Alofisel (darvadstrocel) – development code: Cx601 – for the treatment of complex perianal fistulas in patients with non-active or mildly active luminal Crohn’s disease (CD).3 This product is indicated for the treatment of patients who have shown an inadequate response to at least one existing medicinal treatment.3
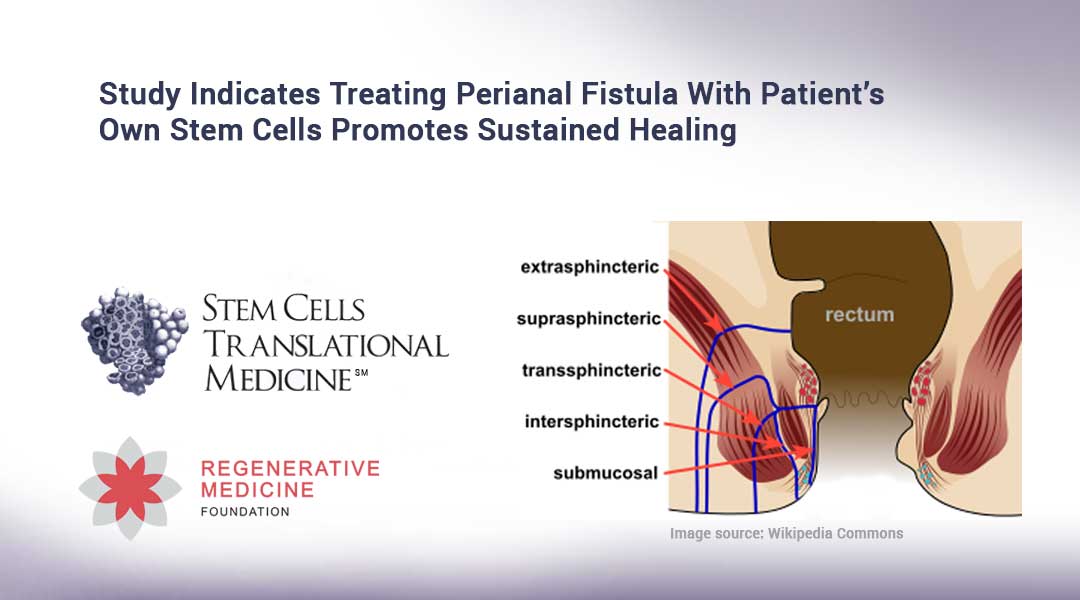
CD is a chronic inflammatory disease of the digestive system,4 which affects an estimated 70,700 people in Japan.5 People living with CD may experience complex perianal fistulas, which can cause intense pain, bleeding, swelling, infection, and anal discharge.6 Despite medical and surgical advancements, complex perianal fistulas in CD remain challenging for clinicians to treat.7 In a survey commissioned and conducted by the European Federation of Crohn’s & Ulcerative Colitis Associations (“EFCCA”), in collaboration with Takeda, CD patients living with perianal fistulas reported a negative impact on many aspects of their quality of life, including the ability to do sports, work and employment, dating and sexual life.8 Patients also reported feeling more unhygienic, uncomfortable, and guilty about their condition compared to CD patients without perianal fistulas.8
“We are delighted that Alofisel has been approved in Japan for the treatment of complex perianal fistulas in Crohn’s disease patients. Complex perianal fistulas are a challenging complication of Crohn’s disease, and the approval of the first expanded human allogeneic adipose-derived mesenchymal stem cell therapy in Japan offers a potential cell-mediated closure option to patients who do not respond to conventional or biologic therapies,” said Naoyoshi Hirota, General Manager of Takeda Development Center Japan.
The approval is based on data from two trials, the Japanese Study Darvadstrocel-3002 and the ADMIRE-CD trial, conducted in Europe and Israel:1,9,10
- Study Darvadstrocel-3002 was a Phase 3, multicenter, open-label, uncontrolled study investigating the efficacy (24 and 52 weeks) and safety (156 weeks) of Alofisel for the treatment of complex perianal fistulas in 22 Japanese adult patients with non-active/mildly active luminal CD9,10
- ADMIRE-CD was a randomized, double-blind, controlled, Phase 3 trial investigating the efficacy and safety of Alofisel for the treatment of complex perianal fistulas in 212 adult patients with non-active/mildly active luminal CD.1 A significantly greater proportion of patients in the Alofisel group compared to the control group achieved the primary endpoint of combined remission at a 24 week follow-up (51.5% vs 35.6%; 97.5% CI 0.5-31.2; P =0.021), and this was maintained over 52 weeks (56.3% vs 38.6%; 95% CI 4.2-31.2; P =0.01).11 Alofisel treatment was well-tolerated over 52 weeks, with a similar safety profile in the Alofisel group compared to the control group11
Alofisel is the first expanded human allogeneic adipose-derived mesenchymal stem cell therapy to be approved in Japan. By exhibiting immunomodulatory and local anti-inflammatory effects at the site of inflammation, it offers a new treatment option with the potential of cell-mediated closure for complex perianal fistulas in patients with non-active or mildly active luminal Crohn’s disease who have shown an inadequate response to at least one existing medicinal treatment.1,2,3
It is a made to order therapy that must be administered within 72 hours of manufacture. Takeda has established a manufacturing and logistics system in Japan that enables delivery to medical institutions nationwide under strict temperature control.
About Alofisel
Alofisel is a suspension of allogeneic (or donor-derived) expanded adipose-derived stem cells (eASC) for the treatment of complex perianal fistulas in adult patients with non-active or mildly active luminal CD.2 Alofisel was granted orphan drug designation by the European Commission in 2009,12 the U.S. Food and Drug Administration (FDA) in 2017,13 and the Japanese Ministry of Health, Labour and Welfare on March 13, 2019.14 Alofisel received central marketing authorization approval in the European Union in March 2018 for the treatment of complex perianal fistulas in adult patients with non-active/mildly active luminal Crohn’s disease, when fistulas have shown an inadequate response to at least one conventional or biologic therapy.2 In 2019, darvadstrocel received a Regenerative Medicine Advanced Therapy (RMAT) designation from the U.S. FDA for complex perianal fistulas in adults with CD.15
Therapeutic Indications
Alofisel is approved in the European Union/European Economic Area, Israel, Switzerland and the United Kingdom for the treatment of complex perianal fistulas in adult patients with non-active/mildly active luminal Crohn’s disease, when fistulas have shown an inadequate response to at least one conventional or biologic therapy.2,16,17 Alofisel should be used only after conditioning of the fistulas.2,16,17
In Japan, Alofisel is indicated for the treatment of complex perianal fistulas in patients with non-active or mildly active luminal Crohn’s disease (CD).3 This product is indicated for the treatment of patients who have shown an inadequate response to at least one existing medicinal treatment.3
Important Safety Information2
Contraindications
Hypersensitivity to the active substance, bovine serum or to any of the excipients.
Special warnings and special precautions for use
Darvadstrocel may contain trace amounts of either gentamicin or benzylpenicillin and streptomycin. This should be considered in patients with known hypersensitivity to these classes of antibiotics. Local anesthesia is not recommended due to the unknown effect of local anesthetics on the injected cells.
The injection of any substance other than sodium chloride 9 mg/mL (0.9%) (e.g. hydrogen peroxide, methylene blue, iodine solutions or hypertonic glucose solutions) through the fistula tracts is not allowed before, during, or after the injection of darvadstrocel as this may compromise cell viability.
Darvadstrocel is indicated for injection in the fistula tract tissue only. Darvadstrocel must not be administered using a needle thinner than 22G. Thinner gauge needles can cause cell disruption during injection and may compromise cell viability.
As darvadstrocel is a living stem cell therapy, it cannot be sterilized, and therefore could contain potentially infected biological material, although the risk is considered to be low and controlled in the manufacturing process. Patients should be followed up for potential signs of infection after administration.
Darvadstrocel should only be administered by specialist physicians experienced in the diagnosis and treatment of conditions for which Darvadstrocel is indicated.
Fertility, Pregnancy & Lactation
No data is available from the use of darvadstrocel in pregnant or breast-feeding women. Darvadstrocel is not recommended during pregnancy and in women of childbearing potential who are not using contraception. As a precautionary measure, darvadstrocel is not recommended for administration during breast-feeding.
Adverse reactions include: Common (≥1/100 to <1/10): anal abscess, proctalgia, anal fistula and procedural pain. Conditioning of fistulas has been associated with proctalgia and procedural pain.
| ▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See Section 4.8 of the SmPC for how to report adverse reactions. |
For EU audiences, please see the Summary of Product Characteristics (SmPC) for ALOFISEL®▼.
Please consult with your local regulatory agency for approved labeling in your country.
Disclaimer
Regulatory approval of the product and its use is dependent on the review by Japanese regulatory authorities. The drug information contained herein is intended for the disclosure of Takeda corporate information and is not intended to advertise or promote any prescription drug, including those under development.
Takeda in Gastroenterology
We believe that gastrointestinal (GI) and liver diseases are not just life-disrupting conditions, but diseases that can impact a patient’s quality of life.18,19 Beyond a fundamental need for effective treatment options, we understand that improving patients’ lives also depends on their needs being recognized. With nearly 30 years of experience in gastroenterology, Takeda has made significant strides in addressing GI patient needs with treatments for inflammatory bowel disease (IBD), acid-related diseases, short bowel syndrome (SBS), and motility disorders. We are making significant strides toward closing the gap on new areas of unmet needs for patients who have celiac disease, eosinophilic esophagitis, alpha-1 antitrypsin-associated liver disease and Crohn’s disease, among others. Together with researchers, patient groups and more, we are working to advance scientific research and clinical medicine in GI.
About Takeda Pharmaceutical Company Limited
Takeda Pharmaceutical Company Limited (TSE: 4502/NYSE: TAK) is a global, values-based, R&D-driven biopharmaceutical leader headquartered in Japan, committed to discover and deliver life-transforming treatments, guided by our commitment to patients, our people and the planet. Takeda focuses its R&D efforts on four therapeutic areas: Oncology, Rare Genetic and Hematology, Neuroscience, and Gastroenterology (GI). We also make targeted R&D investments in Plasma-Derived Therapies and Vaccines. We are focusing on developing highly innovative medicines that contribute to making a difference in people’s lives by advancing the frontier of new treatment options and leveraging our enhanced collaborative R&D engine and capabilities to create a robust, modality-diverse pipeline. Our employees are committed to improving quality of life for patients and to working with our partners in healthcare in approximately 80 countries. For more information, visit https://www.takeda.com.
| Media Contacts: | |
| Japanese Media Kazuhisa Kaneko [email protected] +81-3-3278-3099 |
Media outside Japan Luke Willats [email protected] +41-44-555-1145 |
Important Notice
For the purposes of this notice, “press release” means this document, any oral presentation, any question and answer session and any written or oral material discussed or distributed by Takeda Pharmaceutical Company Limited (“Takeda”) regarding this release. This press release (including any oral briefing and any question-and-answer in connection with it) is not intended to, and does not constitute, represent or form part of any offer, invitation or solicitation of any offer to purchase, otherwise acquire, subscribe for, exchange, sell or otherwise dispose of, any securities or the solicitation of any vote or approval in any jurisdiction. No shares or other securities are being offered to the public by means of this press release. No offering of securities shall be made in the United States except pursuant to registration under the U.S. Securities Act of 1933, as amended, or an exemption therefrom. This press release is being given (together with any further information which may be provided to the recipient) on the condition that it is for use by the recipient for information purposes only (and not for the evaluation of any investment, acquisition, disposal or any other transaction). Any failure to comply with these restrictions may constitute a violation of applicable securities laws.
The companies in which Takeda directly and indirectly owns investments are separate entities. In this press release, “Takeda” is sometimes used for convenience where references are made to Takeda and its subsidiaries in general. Likewise, the words “we”, “us” and “our” are also used to refer to subsidiaries in general or to those who work for them. These expressions are also used where no useful purpose is served by identifying the particular company or companies.
Forward-Looking Statements
This press release and any materials distributed in connection with this press release may contain forward-looking statements, beliefs or opinions regarding Takeda’s future business, future position and results of operations, including estimates, forecasts, targets and plans for Takeda. Without limitation, forward-looking statements often include words such as “targets”, “plans”, “believes”, “hopes”, “continues”, “expects”, “aims”, “intends”, “ensures”, “will”, “may”, “should”, “would”, “could” “anticipates”, “estimates”, “projects” or similar expressions or the negative thereof. These forward-looking statements are based on assumptions about many important factors, including the following, which could cause actual results to differ materially from those expressed or implied by the forward-looking statements: the economic circumstances surrounding Takeda’s global business, including general economic conditions in Japan and the United States; competitive pressures and developments; changes to applicable laws and regulations; the success of or failure of product development programs; decisions of regulatory authorities and the timing thereof; fluctuations in interest and currency exchange rates; claims or concerns regarding the safety or efficacy of marketed products or product candidates; the impact of health crises, like the novel coronavirus pandemic, on Takeda and its customers and suppliers, including foreign governments in countries in which Takeda operates, or on other facets of its business; the timing and impact of post-merger integration efforts with acquired companies; the ability to divest assets that are not core to Takeda’s operations and the timing of any such divestment(s); and other factors identified in Takeda’s most recent Annual Report on Form 20-F and Takeda’s other reports filed with the U.S. Securities and Exchange Commission, available on Takeda’s website at: https://www.takeda.com/investors/ reports/sec-filings/ or at www.sec.gov. Takeda does not undertake to update any of the forward-looking statements contained in this press release or any other forward-looking statements it may make, except as required by law or stock exchange rule. Past performance is not an indicator of future results and the results or statements of Takeda in this press release may not be indicative of, and are not an estimate, forecast, guarantee or projection of Takeda’s future results.
References
1 Panés J, García-Olmo D, Van Assche G, et al. ADMIRE CD Study Group Collaborators. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;24;388(10051):1281-90.
2 Alofisel Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/alofisel-epar-product-information_en.pdf. Last updated November 24, 2020. Last accessed September 2021.
3 Alofisel Japanese package insert. Available upon request.
4 Xavier RJ and Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007; 448: 427-434.
5 Murakami Y, Nishiwaki Y, Oba MS, et al. Estimated prevalence of ulcerative colitis and Crohn’s disease in Japan in 2014: an analysis of a nationwide survey. J Gastroenterol. 2019; 54:1070-1077.
6 Aguilera-Castro L, Ferre-Aracil C, Garcia-Garcia-de-Paredes A, et al. Management of complex perianal Crohn’s disease. Ann Gastroenterol. 2017;30:33-44.
7 Geltzeiler C, Wieghard N and Tsikitis V. Recent developments in the surgical management of perianal fistula for Crohn’s disease. Ann Gastroenterol. 2014; 27(4): 320-330.
8 Spinelli A, Yanai H, Lonnfors S, et al. The impact of perianal fistula in Crohn’s disease on quality of life: results of a
patient survey conducted in Europe. Presented virtually at ECCO, July 2021.
9 Phase 3 Study of Cx601 in Subjects With Complex Perianal Fistulising Crohn’s Disease. Available at https://clinicaltrials.gov/ct2/show/NCT03706456. Last updated: October 5, 2020. Last accessed September 2021.
10 JAPIC Clinical Trials Information. JapicCTI-184145. Available at https://www.clinicaltrials.jp/cti-user/trial/ShowDirect.jsp?clinicalTrialId=27403. Last updated July 26, 2021. Last accessed September 2021.
11 Panés J, García-Olmo D, Van Assche G, et al. ADMIRE CD Study Group Collaborators. Long-term Efficacy and Safety of Stem Cell Therapy (Cx601) for Complex Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology. 2018; 154(5): 1334-1342.
12 Alofisel European public assessment report summary. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/alofisel. Last updated: November 24, 2020. Last accessed September 2021.
13 U.S. Food & Drug Administration Orphan Drug Designation & Approval. Available at: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=373012. Last updated October 18, 2017. Last accessed September 2021.
14 Orphan drug list. Available at:
https://www.nibiohn.go.jp/nibio/part/promote/orphan_support/index.html#hyodata00 . Last updated June 23, 2020.
Last accessed September 2021.
15 Data on file. RMAT Designation Letter, May 2019. Takeda Pharmaceuticals USA, Inc.
16 Ministry of Health Israel. The Israeli Drug Registry. Alofisel. Available at: https://data.health.gov.il/drugs/alonim/Rishum_18_484505220.pdf. Last updated December 2020. Last accessed September 2021.
17 Swissmedic. Alofisel®, Suspension zur Injektion (Darvadstrocelum). Available at https://www.swissmedic.ch/swissmedic/en/home/humanarzneimittel/authorisations/new-medicines/alofisel_suspensionzurinjektion_darvadstrocelum.html. Last updated December 27, 2018. Last accessed September 2021.
18 Center for Drug Evaluation and Research (CDER) & the FDA. The Voice of the patient/functional gastrointestinal disorder. 2016. Available at: https://www.fda.gov/media/95140/download. Last accessed September 2021.
19 Jones R, Hunt C, Stevens R, et al. Management of common gastrointestinal disorders: quality criteria based on patients’ views and practice guidelines. Br J Gen Pract. 2009;59:e199-208.