By Nate MacKay – Courthouse News Service (Link to article)
The implanted tissues successfully restored walking abilities in 80% of the lab models with chronic paralysis.
(CN) — Researchers engineered and implanted functional human spinal cord tissues that showed success in treating paralysis in lab models.
The study, published Monday in the journal Advanced Science, comes from researchers at Sagol Center for Regenerative Biotechnology at Tel Aviv University. Using human materials and cells, the researchers developed three-dimensional spinal cord tissues designed to avoid rejection by the patient’s body after implantation. In the lab models, 100% of the recently paralyzed individuals regained their ability to walk. Among the lab models with chronic paralysis, 80% could walk after receiving the implant.
“Our goal is to produce personalized spinal cord implants for every paralyzed person, enabling regeneration of the damaged tissue with no risk of rejection,” said Tal Dvir, leader of the research team and head of the Sagol Center for Regenerative Biotechnology, in a statement accompanying the study.
The process begins with a small biopsy of the patient’s belly fat tissue. The researchers conduct genetic engineering to separate the cells and revert them to a state resembling embryonic stem cells, which can become any type of cell in the body.
The tissue from the biopsy gives researchers an extracellular matrix, which they use to produce a personalized hydrogel. This hydrogel is patient-specific to sidestep an immune response to or rejection of the tissue after it is implanted. The researchers encapsulate the reverted stem cells in the hydrogel, which Dvir said “mimics the embryonic development of the spinal cord.” The cells develop into a three-dimensional implant that has the network of specialized brain cells found in the spine that help facilitate movement.
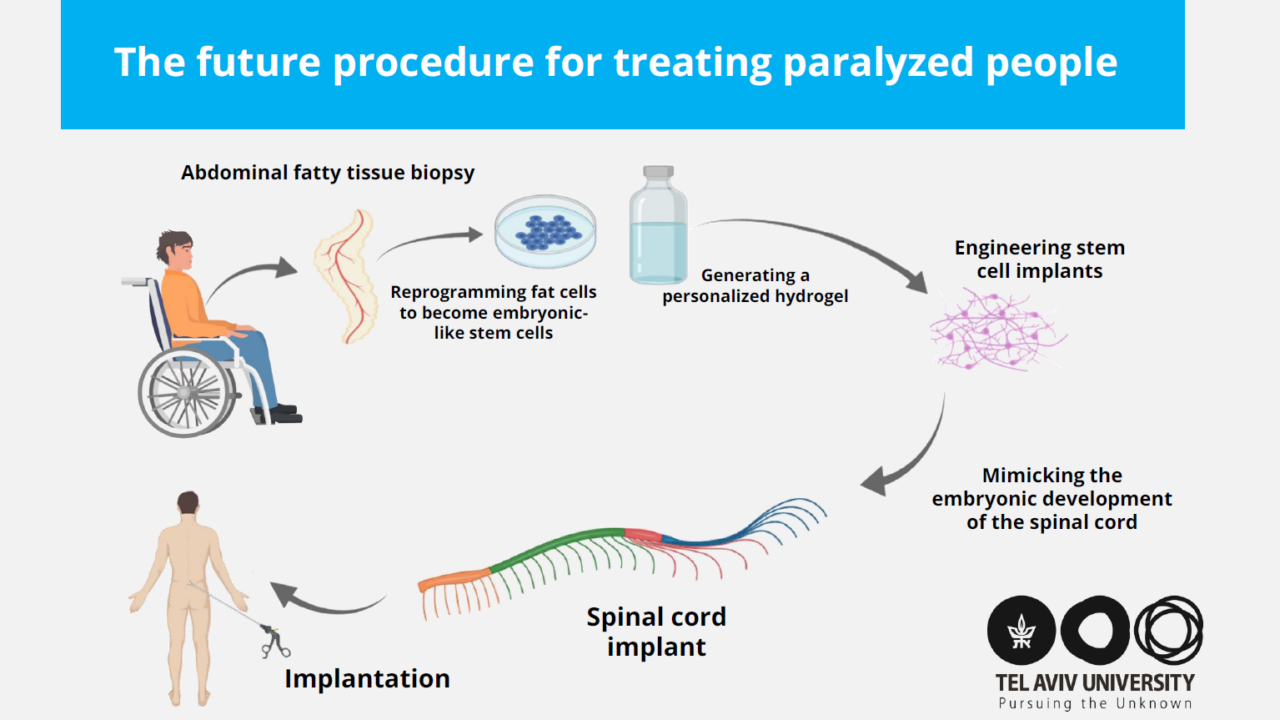
Visualization of the next stage of the research — human spinal cord implants for treating paralysis. (Sagol Center for Regenerative Biotechnology)
Given the implants’ success in the laboratory setting, the scientists are hopeful that within a few years the technology can be deployed to engineer tissues that can help individuals whose spinal injuries or other conditions have left them paralyzed with few treatment options at their disposal.
“The model animals underwent a rapid rehabilitation process, at the end of which they could walk quite well,” Dvir said. “This is the first instance in the world in which implanted engineered human tissues have generated recovery in an animal model for long-term chronic paralysis — which is the most relevant model for paralysis treatments in humans.”
As of a 2013 study from the National Institutes of Health, approximately 5.4 million people were living with paralysis in the United States alone, and 72% of them were younger than 65. The leading causes of paralysis in the United States are stroke, spinal cord injury and multiple sclerosis.
In 2019, Dvir co-founded Matricelf, a regenerative medicine company through which he hopes to make treatments like the spinal cord implants commercially available to patients.
“We hope to reach the stage of clinical trials in humans within the next few years, and ultimately get these patients back on their feet,” Dvir said. “The company’s preclinical program has already been discussed with the FDA. Since we are proposing an advanced technology in regenerative medicine, and since at present there is no alternative for paralyzed patients, we have good reason to expect relatively rapid approval of our technology.”