By Michael Hiltzik | Business Columnist | Los Angeles Times
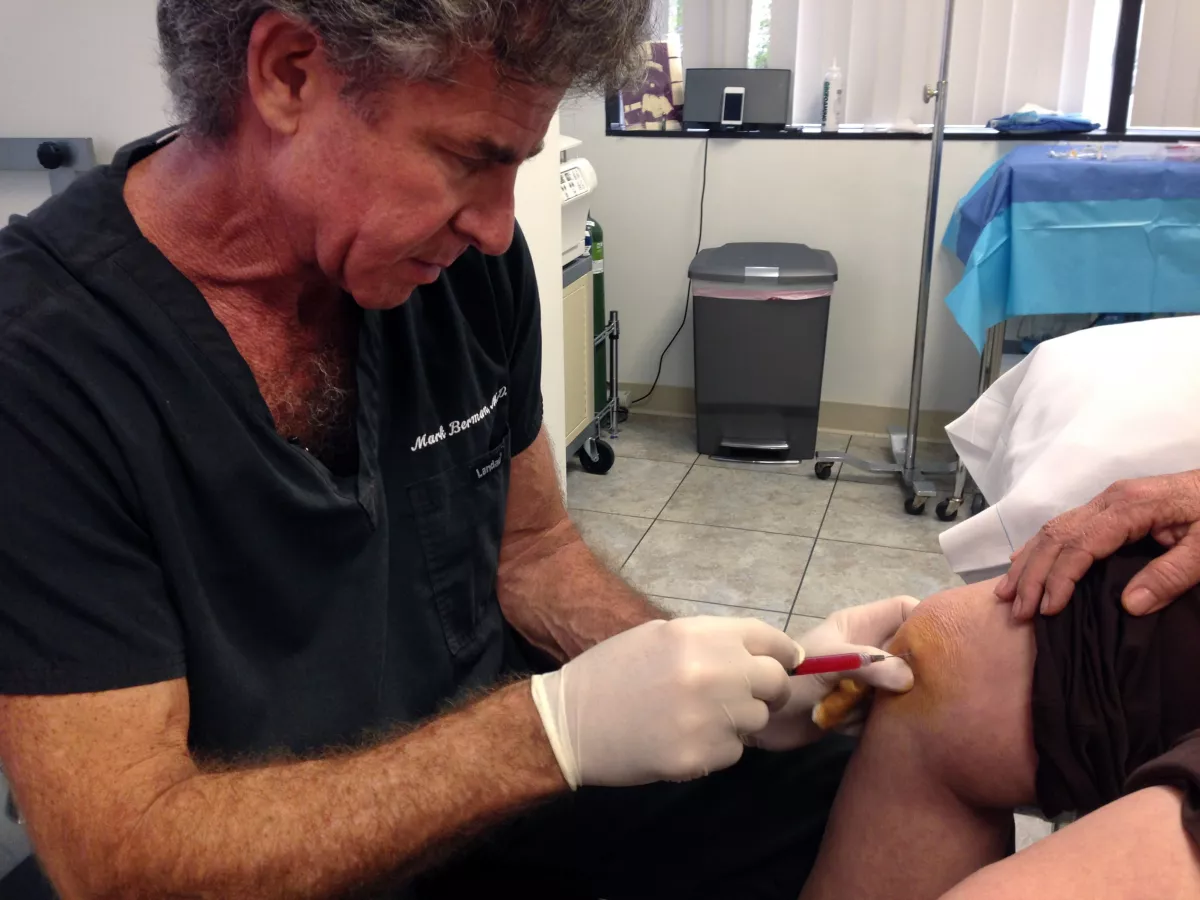
A federal judge in Riverside declared a California stem cell treatment firm to be exempt from Food and Drug Administration regulations, opening the door to the further proliferation of clinics offering therapies the FDA says are scientifically unproven and potentially dangerous.
In the ruling issued late Tuesday, Judge Jesus G. Bernal of Riverside declined to block California Stem Cell Treatment Center from continuing to offer purported stem cell treatments to customers.
Bernal accepted the center’s position that its treatments qualified for an exception from FDA regulations, in part because they were tantamount to surgical procedures.