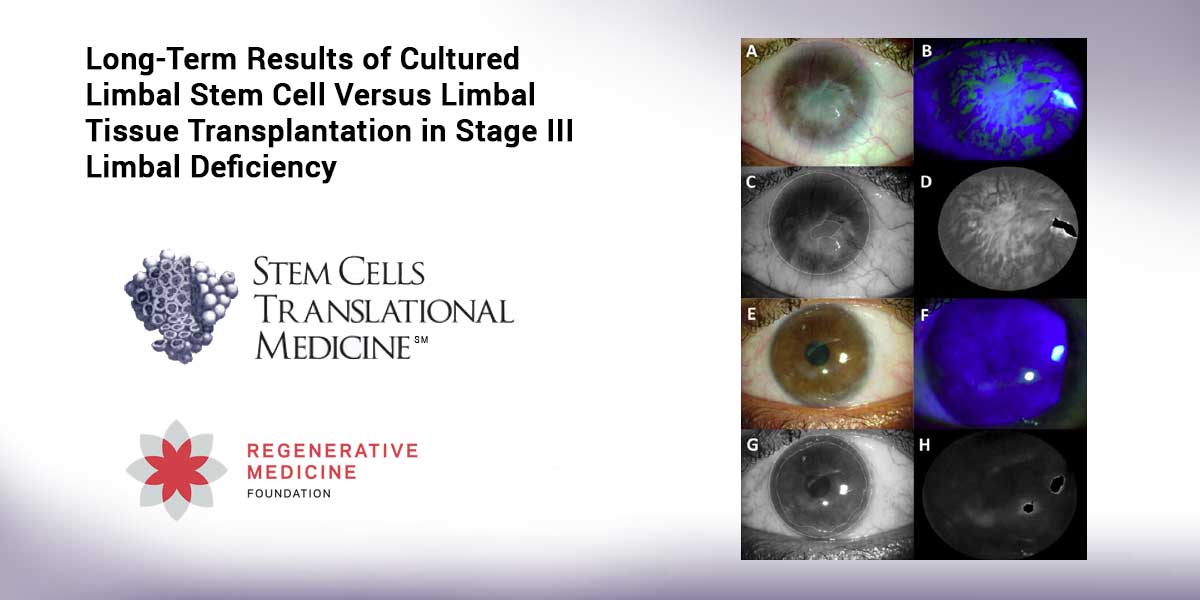
by Admin | Sep 5, 2019 | News & Opinions
Durham, NC – Results of a phase II clinical trial released today in STEM CELLS Translational Medicine (SCTM) indicate that a limbal stem cell (LSC) transplantation is superior to a tissue graft in treating limbal stem cell deficiency syndrome (LSCD). This could result...

by Admin | Sep 4, 2019 | News & Opinions
Stemell sold stem cell products without required FDA approval The U.S. Food and Drug Administration has warned Stemell, Inc. (Stemell), of San Juan Capistrano, California, and its president and Chief Executive Officer, Peyman Taeidi, Ph.D., for manufacturing and...
![Woman is first to receive cornea made from ‘reprogrammed’ stem cells]()
by Admin | Sep 2, 2019 | News & Opinions
Image Caption: The transparent cornea protects the eye from damage. Image Credit: Ralph C. Eagle Jnr/Science Photo Library The Japanese woman’s vision has improved since the transplant, say her doctors. By: David Cyranoski – Nature.com A Japanese woman in her...
by Admin | Sep 1, 2019 | News & Opinions
Clinics claim that expensive stem cell therapies can help patients with dementia, autism, multiple sclerosis and even cerebral palsy – and crowdfunding campaigns to pay for the treatments are increasingly common. But are the patients and the donors being misled...
by Admin | Aug 28, 2019 | News & Opinions
Washington, D.C. – August 27, 2019 – The Alliance for Regenerative Medicine (ARM), the international advocacy organization representing the cell and gene therapy and broader regenerative medicine sector, today released a Therapeutic Developers’ Statement of...





